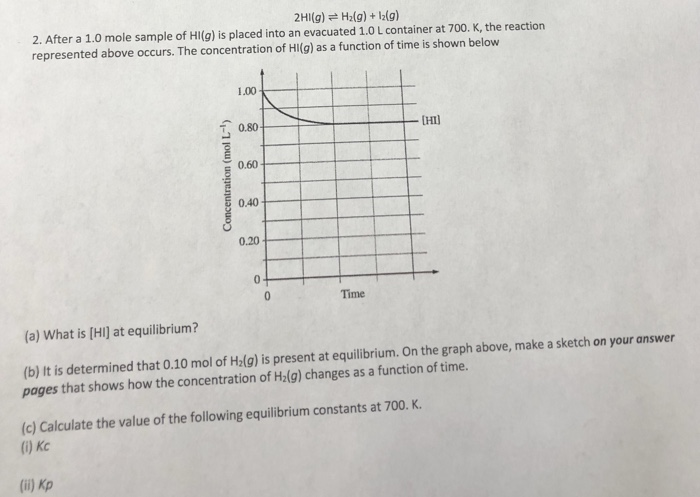
20+ pages after 1.0 mole sample of hi 3mb. Leya 22K to be as exact as i can it is all of them they all work together to make water good. 20 mol -- I got. H2g 12g 1. Read also sample and learn more manual guide in after 1.0 mole sample of hi 1 060 Concentration mol L- 040 020 b.
Request an answer from our educators and we will get to it right away. Jump To Question Problem 1 Problem 2 Problem 3 Problem 4 Problem 5 Problem 6 Problem 7 Problem 8.

Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy
| Title: Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy |
| Format: PDF |
| Number of Pages: 311 pages After 1.0 Mole Sample Of Hi |
| Publication Date: June 2017 |
| File Size: 3mb |
| Read Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy |
 |
Calculate the pH of 10 L of 010 M pyridine solution to which 03 mol of pyridinium chloride C_5H_5.
K the reaction represented occurs. Pages 19 This preview shows page 10 - 15 out of 19 pages. A 10 mole sample of HNO3 is added to water. After a 10 mole sample of HI g is placed into an evacuated 10 L container at 700K the reaction below occurs. When equilibrium is reached what is the molar concentration of I2. Write the expression for the equilibrium constant Kc for the reaction.

142 U14 At 2800 K A 1 0 Mole Sample Of Co In A One Litre Container Is 50 Deposed To Carbon Monoxide And Oxygen At Equilibrium 2102 Juoz 200 9 2co G O2 G
| Title: 142 U14 At 2800 K A 1 0 Mole Sample Of Co In A One Litre Container Is 50 Deposed To Carbon Monoxide And Oxygen At Equilibrium 2102 Juoz 200 9 2co G O2 G |
| Format: PDF |
| Number of Pages: 302 pages After 1.0 Mole Sample Of Hi |
| Publication Date: May 2021 |
| File Size: 3.4mb |
| Read 142 U14 At 2800 K A 1 0 Mole Sample Of Co In A One Litre Container Is 50 Deposed To Carbon Monoxide And Oxygen At Equilibrium 2102 Juoz 200 9 2co G O2 G |
 |

A 3 Mole Sample Of A Triatomic Ideal Gas At 300 K Is Allowed To Expand Under Adiabatic Reversible Condition From 5l To 40 L The Value Of Deltah Is
| Title: A 3 Mole Sample Of A Triatomic Ideal Gas At 300 K Is Allowed To Expand Under Adiabatic Reversible Condition From 5l To 40 L The Value Of Deltah Is |
| Format: eBook |
| Number of Pages: 301 pages After 1.0 Mole Sample Of Hi |
| Publication Date: March 2020 |
| File Size: 1.8mb |
| Read A 3 Mole Sample Of A Triatomic Ideal Gas At 300 K Is Allowed To Expand Under Adiabatic Reversible Condition From 5l To 40 L The Value Of Deltah Is |
 |

At 87 C The Following Equilibrium Is Established H 2 G S S Harrh 2 S S G K P 7xx10 2 If 0 50 Mole Of Hydrogen And 1 0 Mole Of Sulphuur Are Heated To 87 C And 2 0 Atm The Equilibrium Gases Mixture
| Title: At 87 C The Following Equilibrium Is Established H 2 G S S Harrh 2 S S G K P 7xx10 2 If 0 50 Mole Of Hydrogen And 1 0 Mole Of Sulphuur Are Heated To 87 C And 2 0 Atm The Equilibrium Gases Mixture |
| Format: PDF |
| Number of Pages: 295 pages After 1.0 Mole Sample Of Hi |
| Publication Date: February 2018 |
| File Size: 810kb |
| Read At 87 C The Following Equilibrium Is Established H 2 G S S Harrh 2 S S G K P 7xx10 2 If 0 50 Mole Of Hydrogen And 1 0 Mole Of Sulphuur Are Heated To 87 C And 2 0 Atm The Equilibrium Gases Mixture |
 |
S Wongchemistry Weebly Uploads 5 1 3 6 5136424 Ap Frqs Gaseous Equilibrium Ans Pdf
| Title: S Wongchemistry Weebly Uploads 5 1 3 6 5136424 Ap Frqs Gaseous Equilibrium Ans Pdf |
| Format: PDF |
| Number of Pages: 190 pages After 1.0 Mole Sample Of Hi |
| Publication Date: June 2020 |
| File Size: 1.7mb |
| Read S Wongchemistry Weebly Uploads 5 1 3 6 5136424 Ap Frqs Gaseous Equilibrium Ans Pdf |
 |

1 9701 W16 Qp 23 Moles And Stoichiometry Moles And Concentration
| Title: 1 9701 W16 Qp 23 Moles And Stoichiometry Moles And Concentration |
| Format: ePub Book |
| Number of Pages: 137 pages After 1.0 Mole Sample Of Hi |
| Publication Date: June 2021 |
| File Size: 1.5mb |
| Read 1 9701 W16 Qp 23 Moles And Stoichiometry Moles And Concentration |
 |

142 U14 At 2800 K A 1 0 Mole Sample Of Co In A One Litre Container
| Title: 142 U14 At 2800 K A 1 0 Mole Sample Of Co In A One Litre Container |
| Format: eBook |
| Number of Pages: 179 pages After 1.0 Mole Sample Of Hi |
| Publication Date: February 2017 |
| File Size: 1.4mb |
| Read 142 U14 At 2800 K A 1 0 Mole Sample Of Co In A One Litre Container |
 |

Calculate The Enthalpy Change Of 1 Mole Of Reaction Na S 1 2 Br 2 G Rarrnabr S In Kcal Given Delta H Sub Na 137 Kj Mole 1 Deltah Bond Dissociation Br 2 G 144 Kj Mole 1 Delta H 1 St Ionisation Na G 496 Kj Mole
| Title: Calculate The Enthalpy Change Of 1 Mole Of Reaction Na S 1 2 Br 2 G Rarrnabr S In Kcal Given Delta H Sub Na 137 Kj Mole 1 Deltah Bond Dissociation Br 2 G 144 Kj Mole 1 Delta H 1 St Ionisation Na G 496 Kj Mole |
| Format: eBook |
| Number of Pages: 340 pages After 1.0 Mole Sample Of Hi |
| Publication Date: December 2020 |
| File Size: 810kb |
| Read Calculate The Enthalpy Change Of 1 Mole Of Reaction Na S 1 2 Br 2 G Rarrnabr S In Kcal Given Delta H Sub Na 137 Kj Mole 1 Deltah Bond Dissociation Br 2 G 144 Kj Mole 1 Delta H 1 St Ionisation Na G 496 Kj Mole |
 |
2hi G H2 G 12g 2 After A 1 0 Mole Sample Of Hl G Chegg
| Title: 2hi G H2 G 12g 2 After A 1 0 Mole Sample Of Hl G Chegg |
| Format: PDF |
| Number of Pages: 234 pages After 1.0 Mole Sample Of Hi |
| Publication Date: April 2021 |
| File Size: 5mb |
| Read 2hi G H2 G 12g 2 After A 1 0 Mole Sample Of Hl G Chegg |
 |

A Le 9 0 5 Mole Of H2 G And 1 0 Mole Of Hi But Nol Are Added To A 1 0 L Vessel And Allowed To Reach Equilibrium According To The Following Reaction H 1
| Title: A Le 9 0 5 Mole Of H2 G And 1 0 Mole Of Hi But Nol Are Added To A 1 0 L Vessel And Allowed To Reach Equilibrium According To The Following Reaction H 1 |
| Format: ePub Book |
| Number of Pages: 235 pages After 1.0 Mole Sample Of Hi |
| Publication Date: June 2020 |
| File Size: 2.1mb |
| Read A Le 9 0 5 Mole Of H2 G And 1 0 Mole Of Hi But Nol Are Added To A 1 0 L Vessel And Allowed To Reach Equilibrium According To The Following Reaction H 1 |
 |

If 1 0 Mole Of I 2 Is Introduced In A 1 0 Litre Flask At 1000 K K C 10 6 Which One Is Correct
| Title: If 1 0 Mole Of I 2 Is Introduced In A 1 0 Litre Flask At 1000 K K C 10 6 Which One Is Correct |
| Format: eBook |
| Number of Pages: 233 pages After 1.0 Mole Sample Of Hi |
| Publication Date: May 2019 |
| File Size: 3.4mb |
| Read If 1 0 Mole Of I 2 Is Introduced In A 1 0 Litre Flask At 1000 K K C 10 6 Which One Is Correct |
 |
1 00 After A 1 0 Mole Sample Of Hi G Is Placed Into Chegg
| Title: 1 00 After A 1 0 Mole Sample Of Hi G Is Placed Into Chegg |
| Format: ePub Book |
| Number of Pages: 176 pages After 1.0 Mole Sample Of Hi |
| Publication Date: December 2017 |
| File Size: 2.1mb |
| Read 1 00 After A 1 0 Mole Sample Of Hi G Is Placed Into Chegg |
 |
2Na3PO4 3CaCl2 ---- Ca3 PO42 6NaCl how many moles of CaCl2. If x is the equilibrium concentration of 1g the correct equilibrium constant expression is x05 - x 1 1 2x. 10 L container at 700K the reaction below occurs.
Here is all you have to to learn about after 1.0 mole sample of hi A 10-mole sample of krypton gas has a mass of. The concentration of HI. K the reaction represented above occurs. Calculate the enthalpy change of 1 mole of reaction na s 1 2 br 2 g rarrnabr s in kcal given delta h sub na 137 kj mole 1 deltah bond dissociation br 2 g 144 kj mole 1 delta h 1 st ionisation na g 496 kj mole if 1 0 mole of i 2 is introduced in a 1 0 litre flask at 1000 k k c 10 6 which one is correct 142 u14 at 2800 k a 1 0 mole sample of co in a one litre container is 50 deposed to carbon monoxide and oxygen at equilibrium 2102 juoz 200 9 2co g o2 g at 87 c the following equilibrium is established h 2 g s s harrh 2 s s g k p 7xx10 2 if 0 50 mole of hydrogen and 1 0 mole of sulphuur are heated to 87 c and 2 0 atm the equilibrium gases mixture a 3 mole sample of a triatomic ideal gas at 300 k is allowed to expand under adiabatic reversible condition from 5l to 40 l the value of deltah is 1 9701 w16 qp 23 moles and stoichiometry moles and concentration After a 10 mole sample of HIg is placed into an evacuated 10 L container at 700.
